1. Common salt besides being used in the kitchen can also be used as the raw material for making:
(i) washing soda
(ii) bleaching powder
(iii) baking soda
(iv) slaked lime
(i) washing soda
(ii) bleaching powder
(iii) baking soda
(iv) slaked lime
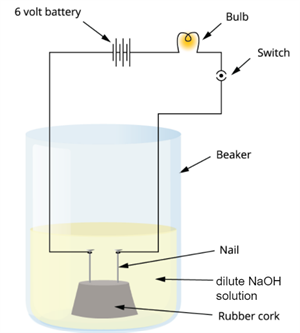
2. In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus
(Figure 2.1) was set up. Which among the following statement(s) is(are) correct?
1. Bulb will not glow because the electrolyte is not acidic
2. Bulb will glow because \(NaOH\) is a strong base and furnishes ions for conduction.
3. Bulb will not glow because the circuit is incomplete .
4. Bulb will not glow because it depends upon the type of electrolytic solution
1. Bulb will not glow because the electrolyte is not acidic
2. Bulb will glow because \(NaOH\) is a strong base and furnishes ions for conduction.
3. Bulb will not glow because the circuit is incomplete .
4. Bulb will not glow because it depends upon the type of electrolytic solution